Update of the ASAS-OMERACT core outcome set for axial spondyloarthritis
Background: The Assessment of SpondyloArthritis international Society-Outcomes Measures in Rheumatology (ASAS-OMERACT) core outcome set (COS) for ankylosing spondylitis (AS) was developed more than two decades ago1 2. Given the progress made since then, both in the knowledge of the disease and in the methodology for developing a COS, ASAS decided to update the original COS for AS into a COS for axial spondyloarthritis (axSpA).
Aim: To update the ASAS-OMERACT core outcome set for AS into a core outcome set for axSpA.
Methodology: Following OMERACT and COMET guidelines, an international working group representing key stakeholders (patients, rheumatologists, health professionals, pharmaceutical industry and drug regulatory agency representatives) defined the core set for axSpA following two consecutive steps. The first step consisted of the selection of the core domains by: i) Identifying candidate domains using a systematic literature review and qualitative studies; ii) A 3-round Delphi survey involving axSpA patients and axSpA experts to select the most relevant domains3; iii) Consensus and voting by ASAS members; iv) Endorsement by OMERACT. Two scenarios are considered based on the type of therapy investigated in the trial: symptom modifying therapies and disease modifying therapies (DMARDs). The second step consisted of the selection of the instruments to assess each of the core domains following a predefined process: i) Identifying candidate instruments using a systematic literature review; ii) Reducing the list of candidate instruments by the working group, iii) Assessing the psychometric properties of all instruments following OMERACT Filter 2.2, iv) Selection of the core instruments by the working group; v) Voting and endorsement by ASAS members.
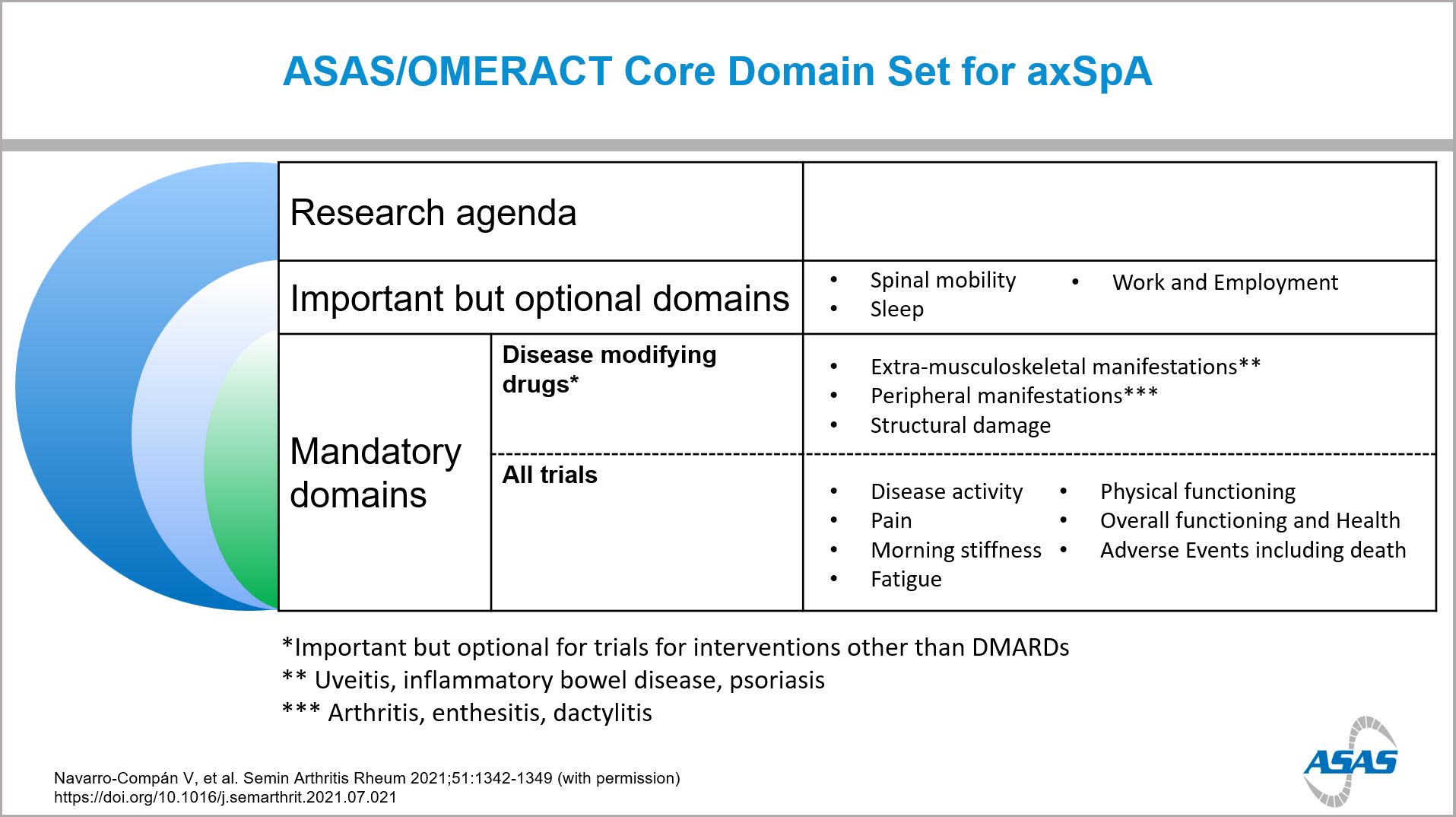
Results: As a first phase of this process the ASAS-OMERACT core domain set was updated and published4. This includes 7 mandatory domains for all studies and 3 additional mandatory domains for studies evaluating DMARDs. The mandatory domains for all trials are: disease activity, pain, morning stiffness, fatigue, physical functioning, overall functioning and health, and adverse events including death. As additional mandatory domains for DMARDs, extra-musculoskeletal manifestations (EMMs), peripheral manifestations and structural damage have been included.
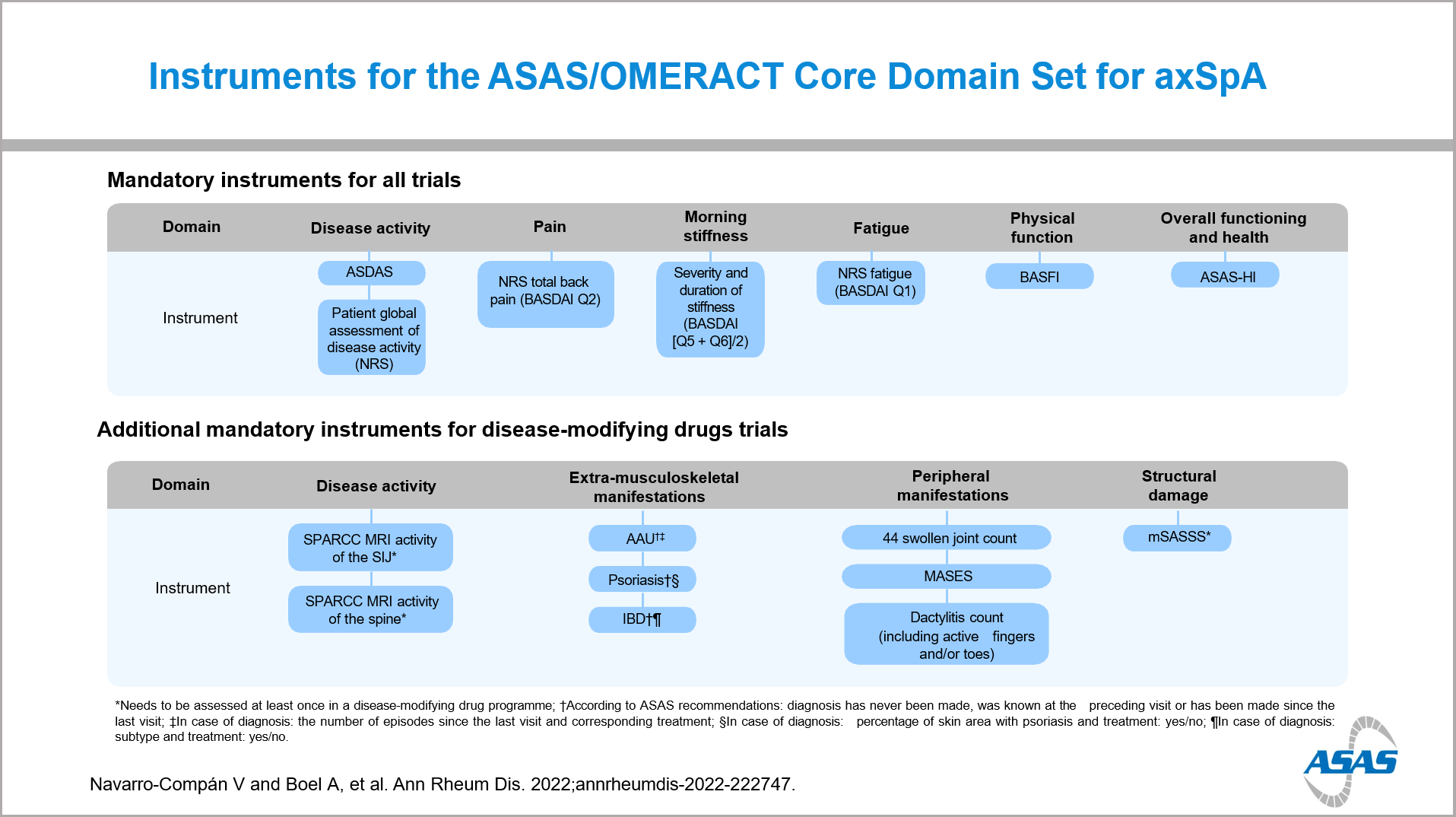
In a second phase, the selection of the instruments was completed5. The updated core set for axSpA includes seven instruments for the domains that are mandatory for all trials: ASDAS and NRS patient global assessment of disease activity; NRS total back pain; average NRS of duration and severity of morning stiffness; NRS fatigue; BASFI; and ASAS Health Index. There are 9 additional instruments considered mandatory for DMARDs trials: MRI activity SPARCC sacroiliac joints and SPARCC spine, uveitis, IBD and psoriasis assessed as recommended by ASAS, 44 swollen joint count, MASES, dactylitis count, and mSASSS. The imaging outcomes are considered mandatory to be included in at least one trial for a drug tested for DMARD-properties. Furthermore, 11 additional instruments were also endorsed by ASAS, which can be used in axSpA trials on top of the core instruments.
Conclusions: The updated ASAS COS for axSpA is a milestone in the field of axSpA. This COS will contribute to ensure that the most relevant aspects of the disease are assessed in all studies and that this is done in a standardised and homogeneous way that will allow comparisons of results across studies. From now on, it should be used in all trials evaluating the efficacy and safety of any type of therapy in patients with axSpA.
Timelines of the project: 2018-2022
Project Team
PIs: Désirée van der Heijde (Leiden, the Netherlands) and Victoria Navarro Compán (Madrid, Spain)
Fellow: Anne de Boel (Leiden, the Netherlands)
Steering committee:
Désirée van der Heijde (Leiden, the Netherlands)
Victoria Navarro Compán (Madrid, Spain)
Anne Boel (Leiden, the Netherlands)
Annelies Boonen (Maastricht, the Netherlands)
Maxime Dougados (Paris, France)
Uta Kiltz (Herne, Germany)
Robert Landewé (Amsterdam, Heerlen, the Netherlands)
Philip Mease (Seattle, USA)
Working group:
Xenofon Baraliakos (Herne, Germany)
Wilson Bautista (Bogotá, Colombia)
Pravina Chiowchanwisawakit (Mahidol, Thailand)
Bassel El-Zorkany (Cairo, Egypt)
Lianne Gensler (San Francisco, USA)
Karl Gaffney (Norwich, United Kingdom)
Nigel Haroon (Toronto, Canada)
Yu Heng Kwan (Singapore, Singapore)
Pedro Machado (London, United Kingdom)
Walter Maksymowych (Edmonton, Canada)
Anna Molto (Paris, France)
Denis Poddubnyy (Berlin, Germany)
Mikhail Protopopov (Berlin, Germany)
Sofia Ramiro (Leiden, Heerlen, the Netherlands)
Salima van Weely (Leiden, the Netherlands)
Marco Garrido Cumbrera (Sevilla, Spain)
Hanne Dagfinrud (Oslo, Norway)
Hilde Carlier (Lilly, USA)
Natasha de Peyrecave (UCB, Belgium)
Lara Fallon (Pfizer, USA)
In-Ho Song (AbbVie, USA)
Beverley Shea (Ontario, Canada)
Publications
- van der Heijde D, Bellamy N, Calin A, et al. Preliminary core sets for endpoints in ankylosing spondylitis. Assessments in Ankylosing Spondylitis Working Group. J Rheumatol 1997;24(11):2225-9. [published Online First: 1998/02/07]
- van der Heijde D, Calin A, Dougados M, et al. Selection of instruments in the core set for DC-ART, SMARD, physical therapy, and clinical record keeping in ankylosing spondylitis. Progress report of the ASAS Working Group. Assessments in Ankylosing Spondylitis. J Rheumatol 1999;26(4):951-4. [published Online First: 1999/05/06]
- Boel A, Navarro-Compan V, Boonen A, et al. Domains to Be Considered for the Core Outcome Set of Axial Spondyloarthritis: Results From a 3-round Delphi Survey. J Rheumatol 2021;48(12):1810-14. doi: 10.3899/jrheum.210206 [published Online First: 20210801]
- Navarro-Compan V, Boel A, Boonen A, et al. The ASAS-OMERACT core domain set for axial spondyloarthritis. Semin Arthritis Rheum 2021;51(6):1342-49. doi: 10.1016/j.semarthrit.2021.07.021 [published Online First: 20210801]
- Navarro-Compan V, Boel A, Boonen A, et al. Instrument selection for the ASAS core outcome set for axial spondyloarthritis. Ann Rheum Dis 2022 doi: 10.1136/annrheumdis-2022-222747 [published Online First: 20220609]