ASDAS calculator
Download the ASAS app with an ASDAS calculator from the App Store or Google Play
or use the online ASDAS calculator below:
Click on the button to load the content from ijon.charite.de.
Ankylosing Spondylitis Disease Activity Score
The Ankylosing Spondylitis Disease Activity Score (ASDAS) is a new composite index to assess disease activity in Ankylosing Spondylitis (AS)1. It combines five disease activity variables with only partial overlap, resulting in one single score with better truth (validity), enhanced discriminative capacity and improved sensitivity to change as compared to single-item variables (1,2).
Table 1. The two ASDAS formulas: ASDAS-CRP (preferred) and ASDAS-ESR (alternative).
ASDAS-CRP
0.12 x Back Pain + 0.06 x Duration of Morning Stiffness + 0.11 x Patient Global + 0.07 x Peripheral Pain/Swelling + 0.58 x Ln(CRP+1)
ASDAS-ESR
0.08 x Back Pain + 0.07 x Duration of Morning Stiffness + 0.11 x Patient Global + 0.09 x Peripheral Pain/Swelling + 0.29 x √(ESR)
ASDAS, Ankylosing Spondylitis Disease Activity Score; √(ESR), square root of the erythrocyte sedimentation rate (mm/h); Ln(CRP+1), natural logarithm of the C-reactive protein (mg/L) + 1. Back pain, patient global, duration of morning stiffness and peripheral pain/swelling are all assessed on a visual analogue scale (from 0 to 10cm) or on a numerical rating scale (from 0 to 10). Back pain, BASDAI question 2: “How would you describe the overall level of AS neck, back or hip pain you have had?”. Duration of morning stiffness, BASDAI question 6: “How long does your morning stiffness last from the time you wake up?”. Patient global: “How active was your spondylitis on average during the last week?” Peripheral pain/swelling, BASDAI question 3: “How would you describe the overall level of pain/swelling in joints other than neck, back or hips you have had?”
The Assessment of SpondyloArthritis international Society (ASAS) membership has selected the ASDAS containing C-reactive protein (CRP, mg/l) as acute phase reactant as the preferred version, and the one with erythrocyte sedimentation rate (ESR, mm/hr) as the alternative version. Apart from the value of CRP or ESR, the four additional self-reported items included in this index are back pain (0-10cm, visual analogue scale [VAS] or 0-10, numerical rating scale [NRS]), duration of morning stiffness (VAS/NRS), peripheral pain/swelling (VAS/NRS) and patient global assessment of disease activity (VAS/NRS) (table 1) (1,2).
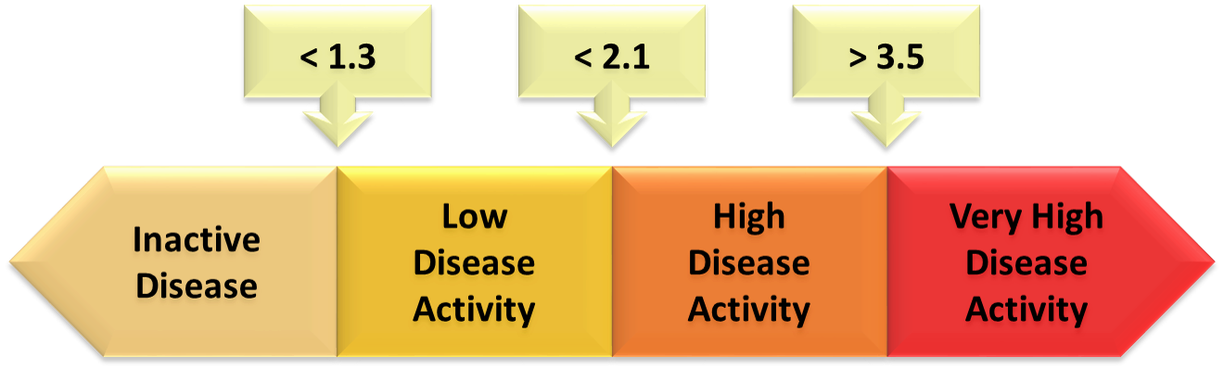
The next step to consolidate the ASDAS as an instrument to measure disease activity in AS was the development of cut-offs for disease activity states and improvement scores. During the 2010 ASAS workshop in Berlin, Germany, cut-offs for the ASDAS were proposed, and cross-validation studies were presented. The methodology and the results were debated by ASAS members and four disease activity states were chosen by consensus: “inactive disease”, “moderate disease activity”, “high disease activity” and “very high disease activity”.
The 3 cut-offs selected to separate these states (figure 1) were: <1.3 between “inactive disease” and “low disease activity”, <2.1 between “moderate disease activity” and “high disease activity”, and >3.5 between “high disease activity” and “very high disease activity” (3,4).
Selected cut-offs for improvement scores (figure 2) were: a change ≥1.1 units for “clinically important improvement” and a change ≥2.0 units for “major improvement” (3).
Figure 1. Selected cut-offs for disease activity states.

At the Outcome Measures in Rheumatology (OMERACT) 10 conference, in Kota Kinabalu, Malaysia, the ASDAS disease activity states and response criteria also obtained the endorsement from OMERACT (5).
Using the ASDAS and the newly validated cut-off values, we hope that clinicians can better assess the effectiveness of treatments and determine whether they are providing clinically meaningful improvement. The higher discriminatory capacity of the ASDAS compared to classical response criteria in AS may have important implications in reducing sample size calculation for clinical trials. The ASDAS will also allow clinicians, investigators, regulators, and patients to continue communicating about treatment response using the same metric.
References
- Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, van der Linden S, van der Heijde D. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18-24. (Toll-Free Link)
- van der Heijde D, Lie E, Kvien TK, Sieper J, van den Bosch F, Listing J, Braun J, Landewé R. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:1811-8. (Toll-Free Link)
- Machado P, Landewé R, Lie E, Kvien TK, Braun J, Baker D, van der Heijde D. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47-53. (Toll-Free Link)
- Machado PM, Landewé R, van der Heijde DV. Assessment of SpondyloArthritis international Society (ASAS). Ankylosing Spondylitis Disease Activity Score (ASDAS): 2018 update of the nomenclature for disease activity states. Ann Rheum Dis. 2018 Feb 16. doi: 10.1136/annrheumdis-2018-213184. [Epub ahead of print] (Toll-Free Link)
- Machado P, Landewé R, van der Heijde D. Endorsement of the definitions of disease activity states and improvement scores for the Ankylosing Spondylitis Disease Activity Score (ASDAS): results from the Outcome Measures in Rheumatology (OMERACT) 10 conference. J Rheumatol 2011;38:1502-6.

